19
The Age of the Sun
Posted by jns on January 19, 2009 This is Sir William Thomson, Baron Kelvin* (1824 – 1907) or, simply, Lord Kelvin as he’s known to us in the physical sciences. This is the same “Kelvin” as in the SI unit “Kelvins”, the degrees of the absolute thermodynamic temperature scale.
This is Sir William Thomson, Baron Kelvin* (1824 – 1907) or, simply, Lord Kelvin as he’s known to us in the physical sciences. This is the same “Kelvin” as in the SI unit “Kelvins”, the degrees of the absolute thermodynamic temperature scale.
The photograph was taken c. 1900 by T. & R. Annan & Sons. I love the title given the photograph by the National Galleries of Scotland: “Sir William Thomson, Baron Kelvin, 1824 – 1907. Scientist, resting on a binnacle and holding a marine azimuth mirror”.
A binnacle, Wikipedia tells me, is a box on the deck of a ship that holds navigational instruments ready for easy reference. One reason Kelvin might be leaning on one is suggested by this bit from the article on “binnacle”
In 1854 a new type of binnacle was patented by John Gray of Liverpool which directly incorporated adjustable correcting magnets on screws or rack and pinions. This was improved again when Lord Kelvin patented in the 1880s another system of compass and which incorporated two compensating magnets.
Kelvin also patented the “marine azimuth mirror” (see the description of “azimuth mirror” from the collection of the Smithsonian National Museum of American History), so the photograph has narrative intent. It seems that Kelvin was an active and successful inventor.
I like this understated biography of Kelvin from the National Galleries of Scotland website (link in first footnote), where it accompanies the photograph:
A child prodigy, William Thomson went to university at the age of eleven. At twenty-two he was appointed Professor of Natural Philosophy in Glasgow where he set up the first physics laboratory in Great Britain and proved an inspiring teacher. He primarily researched thermodynamics and electricity. On the practical side he was involved in the laying of the Atlantic telegraph cable. He was also the partner of a Glasgow firm that made measuring instruments from his own patents.
“He primarily researched thermodynamics and electricity” is a bit of an understatement! Around the time this photograph was taken (c. 1900), Kelvin was pretty much the scientific authority in the world, the great voice of science, the scientist whose opinion on every matter scientific was virtually unassailable.
That unassailability was a huge problem for (at least) Charles Darwin and his theory of common descent by means of natural selection. The crux of the problem was the answer to this question: how old is the Earth?
These days we are quite accustomed to the idea that the Earth is around 4.5 billion years old. At the beginning of the 19th century is was very commonly believed that the Earth was only several thousand years old: Bishop Usher’s calculated date of creation, 23 October 4004 BC, was seen at the time as a scholarly refinement of what everyone already pretty much knew to be true.
Perhaps the big idea growth during the 1800s was the dawning realization of the great antiquity of the Earth. This was accompanied by the realization that fossils might actually be animal remains of some sort; the emergence of geology as a science; and the concept of “uniformitarianism” (an interesting article on the topic), so central to geology, that geological processes in the past, even the deep past, were probably very much like geological processes in action today, so that the geological history of the Earth–and of fossil remains!–could be made sense of.†
Throughout the 19th century discovery after discovery seemed to demand an ever-older Earth. I can imagine that an element of the scientific zeitgeist that precipitated Darwin’s ideas on natural selection as a mechanism for evolution was this growing realization that the Earth might be very, very, very old and that something so remarkably slow as he knew natural selection would be, might be possible. In fact, his ideas went further out on the intellectual limb: he realized that it was necessary that the Earth be much, much older than was currently thought.
In fact, he staked his reputation on the great antiquity of the Earth. This was the critical prediction of his theory, really: the Earth must be vastly older than people thought at the time or else his theory of common descent by natural selection was wrong. It was a bold, seemingly foolhardy claim that he seemed certain to lose.
He was right, of course, but things looked grim at the time and his reasoning was not vindicated by physics until well after his death.
The biggest roadblock to widespread acceptance of the idea of an Earth old enough to allow evolution of humankind through natural selection was none other than Lord Kelvin.
Calculating the age of the Earth looked at the time to be a very challenging problem. But, if there was one thing Kelvin knew, it was that the Earth could not be older than the Sun, and he believed he could calculate the age of the Sun. He was the master of physics, particularly thermodynamics, so all he had to do was add up the sources of energy that contributed to the energy we saw coming from the Sun and figure out how long it might have been going on.
But what were the sources of the Sun’s heat? Kelvin quickly concluded that it could not be any sort of chemical burning, like coal in a fireplace. There simply could not be enough coal. To keep this long story short, Kelvin finally settled on two leading possibilities. One was the energy that came from gravitation contraction of the primordial matter that formed the sun, in which case the sun heated up a great deal originally and then spent eons radiating away its heat. The other possibility that might contribute was the gravitational energy of meteors falling into the sun. (Here’s an interesting and brief exposition of the arguments: S. Gavin, J. Conn, and S. P. Karrer, “The Age of the Sun: Kelvin vs. Darwin“.)
Kelvin published his thoughts in an interesting, and very readable paper, called “On the Age of the Sun’s Heat” , Macmillan’s Magazine, volume 5 (March 5, 1862), pp. 288-293. (html version; pdf version) In the conclusion to that paper Kelvin wrote:
It seems, therefore, on the whole most probable that the sun has not illuminated the earth for 100,000,000 years, and almost certain that he has not done so for 500,000,000 years. As for the future, we may say, with equal certainty, that inhabitants of the earth can not continue to enjoy the light and heat essential to their life for many million years longer unless sources now unknown to us are prepared in the great storehouse of creation.
What an irritant for Darwin! That was not nearly enough time!
These days it is a clichéd joke to say of something that “it violates no known laws of physics”, but that’s the punchline for this entire controversy. Kelvin, naturally, had to search for sources of the Sun’s heat that violated no known laws of physics as they were known at the time, but there were new laws of physics lurking in the wings.
Darwin published On the Origin of Species in 1859. Kelvin published his paper in 1862. Radioactivity was only discovered by Henri Becquerel in 1896. The radioactive decay of elements was not recognized for some time as a possible source of solar energy, but as understanding advanced it was realized that the transmutation of one element into another through radioactive decay involved a loss of mass–the materials before and after could be weighed.
The next domino fell in 1906, a year before Kelvin’s death, when Einstein published his famous equation, 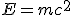 , a consequence of his special theory of relativity. Understanding dawned that the loss of mass was related to a release of energy through the radioactive decay.
, a consequence of his special theory of relativity. Understanding dawned that the loss of mass was related to a release of energy through the radioactive decay.
Our modern knowledge that the sun is powered by nuclear fusion through a process that releases enormous amounts of energy via fusion cycles (interesting, mildly technical paper on its discovery and elucidation) that consume hydrogen atoms to create helium atoms, and then consume those products to produce some heavier elements, was still decades in the future (generally credited to Hans Bethe’s paper published in 1939).
But the message was clear: here was a possible new source of energy for the sun that Kelvin knew nothing about but that could vastly increase the likely age of the Sun.
To finish this part of the story, here is an excerpt from John Gribbin’s The Birth of Time : How Astronomers Measured the Age of the Universe. (New Haven : Yale University Press, 1999. 237 pages.), a fascinating page-turner of a popular science book. (Book note forthcoming.)
If the whole Sun were just slightly radioactive, it could produce the kind of energy that we see emerging from it in the form of heat and light. In 1903, Pierre Curie and his colleague Albert Laborde actually measured the amount of heat released by a gram of radium, and found that it produced enough energy in one hour to raise the temperature of 1.3 grams of water from 0°C to its boiling point. Radium generated enough heat to melt its own weight of ice in an hour–every hour. In July that year, the English astronomer William Wilson pointed out that in that case, if there were just 3.6 grams of radium distributed in each cubic metre of the sun’s volume it would generate enough heat to explain all of the energy being radiated from the Sun’s surface today. It was only later appreciated, as we shall see, that the “enormous energies” referred to by Chamberlin are only unlocked in a tiny region at the heart of the sun, where they produce all of the heat required to sustain the vast bulk of material above them.
The important point, though, is that radioactivity clearly provided a potential source of energy sufficient to explain the energy output of the Sun. In 1903, nobody knew where the energy released by radium (and other radioactive substances) was coming from; but in 1905, another hint at the origin of the energy released in powering both the Sun and radioactive decay came when Albert Einstein published his special theory of relativity, which led to the most famous equation in science,
, relating energy and mass (or rather, spelling out that mass is a form of energy.) This is the ultimate source of energy in radioactive decays, where careful measurements of the weights of all the daughter products involved in such processes have now confirmed that the total weight of all the products is always a little less than the weight of the initial radioactive nucleus–the “lost” mass has been converted directly into energy, in line with Einstein’s equation.
Even without knowing how a star like the Sun might do the trick of converting mass into energy, you can use Einstein’s equation to calculate how much mass has to be used up in this way every second to keep the Sun shining. Overall, about 5 million tonnes of mass have to be converted into pure energy each second to keep the sun shining. This sounds enormous, and it is, by everyday standards–roughly the equivalent of turning five million large elephants into pure energy every second. But the Sun is so big that it scarcely notices this mass loss. If it has indeed been shining for 4.5 billion years, as the radiometric dating of meteorite samples implies, and if it has been losing mass at this furious rate for all that time, then its overall mass has only diminished by about 4 percent since the Solar System formed.
By 1913, Rutherford was commenting that “at the enormous temperatures of the sun, it appears possible that a process of transformation may take place in ordinary elements analogous to that observed in the well-known radio-elements,” and added, “the time during which the sun may continue to emit heat at the present rate may be much longer than the value computed from ordinary dynamical data [the Kelvin-Helmholtz timescale].” [pp. 36--38]
Kelvin was not always right.
———-
* I’ve taken this image from the Flickr Commons set uploaded by the National Galleries of Scotland, and cropped it some from the original: Source and National Galleries page.
† The history of these ideas in the context of geology as an emerging science is very ably traced in Simon Winchester’s The Map that Changed the World : William Smith and the Birth of Modern Geology (my book note).