03
Easy Entropy
Posted by jns on 3 February 2008Thermodynamics is the theory that deals with heat and heat flow without reference to the atomic theory; it was developed at the same time as the steam engine and the family resemblances are striking. All concepts about temperature and pressure in terms of our perceptions of atomic or molecular motion came later and properly belong in the discipline known as Statistical Mechanics.
Awhile back I read this bit in the referenced book and thought it shed a lot of light on the idea of entropy, about which more after the excerpt.
Clausius saw that something was being conserved in Carnot’s [concept of a] perfectly reversible engine; it was just something other than heat. Clausius identified that quantity, and he gave it the name entropy. He found that if he defined entropy as the heat flow from a body divided by its absolute temperature, then the entropy changes in a perfectly reversible engine would indeed balance out. As heat flowed from the boiler to the steam, the boiler’s entropy was reduced. As heat flowed into the condenser coolant, the coolant’s entropy increased by the same amount.
No heat flowed as steam expanded in the cylinder or, as condensed water, it was compressed back to the boiler pressure. Therefore the entropy changed only when heat flowed to and from the condenser and the boiler and the net entropy was zero.
If the engine was perfectly reversible, it and the surroundings with which it interacted remained unchanged after each cycle of the engine. Under his definition of entropy Clausius was able to show that every thing Carnot had claimed was true, except that heat was conserved in his engine.
Once Carnot’s work had been relieved of that single limitation, Clausius could reach another important result: the efficiency of a perfectly reversible heat engine depends upon nothing other than the temperature of the boiler and the temperature of the condenser.
[John H. Lienhard, How Invention Begins (Oxford : Oxford University Press, 2006) p. 90]
Amazing conclusion #1: virtually everything about heat flowing from a warm place to a cool place depends only on the difference in temperature between the warm place and the cool place.
Amazing conclusion #2: there is an idea, call it entropy, that encapsulates #1. If we let 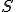 stand for entropy, and
stand for entropy, and 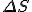 stand for the change in entropy, then we can write
stand for the change in entropy, then we can write
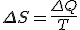
This is Clausius’ definition in symbols.
Now, there’s a lot of philosophical, interpretative baggage that travels with the idea of entropy, but if you can keep this simple approach in mind you can save a lot of heartburn pondering the deeper meaning of entropy and time’s arrow and the heat-death of the universe.
Entropy is an accounting tool. When heat flows between the hot place and the cold place, at best, if you allow it very carefully, you may be able to reverse the process but you will never do better, which means that you will never find the cold place getting colder than originally, nor the hot place getting hotter than originally, no matter what you do, unless you put still more heat into the system.
That’s one version of the notorious “Second Law of Thermodynamics”. There are a number of other forms.
For instance, another way of saying was I just said: entropy never decreases. There, thermodynamic accounting made easy.
Another one that’s useful: if you construct a device that uses heat flowing from a hot place to a cold place to do mechanical work — say, in a steam engine — some of the heat is always wasted, i.e., it goes into increasing entropy. Put another way: heat engines are never 100% efficient, not because we can’t build them but because it is physically impossible.
Think for a moment and you’ll see that the implication of this latter form of the Second Law of Thermodynamics is a statement that perpetual motion machines are impossible. They just are, not because a bunch of physicists though it might be a good idea to say it’s impossible, but because they are. That’s the way the universe is made.
Entropy needn’t be scary.
———-
* 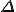 is a general purpose symbol often used to indicate a change in the quantity represented by the letter following it.
is a general purpose symbol often used to indicate a change in the quantity represented by the letter following it.