Feb
03
Posted by jns on
February 3, 2008
Thermodynamics is the theory that deals with heat and heat flow without reference to the atomic theory; it was developed at the same time as the steam engine and the family resemblances are striking. All concepts about temperature and pressure in terms of our perceptions of atomic or molecular motion came later and properly belong in the discipline known as Statistical Mechanics.
Awhile back I read this bit in the referenced book and thought it shed a lot of light on the idea of entropy, about which more after the excerpt.
Clausius saw that something was being conserved in Carnot’s [concept of a] perfectly reversible engine; it was just something other than heat. Clausius identified that quantity, and he gave it the name entropy. He found that if he defined entropy as the heat flow from a body divided by its absolute temperature, then the entropy changes in a perfectly reversible engine would indeed balance out. As heat flowed from the boiler to the steam, the boiler’s entropy was reduced. As heat flowed into the condenser coolant, the coolant’s entropy increased by the same amount.
No heat flowed as steam expanded in the cylinder or, as condensed water, it was compressed back to the boiler pressure. Therefore the entropy changed only when heat flowed to and from the condenser and the boiler and the net entropy was zero.
If the engine was perfectly reversible, it and the surroundings with which it interacted remained unchanged after each cycle of the engine. Under his definition of entropy Clausius was able to show that every thing Carnot had claimed was true, except that heat was conserved in his engine.
Once Carnot’s work had been relieved of that single limitation, Clausius could reach another important result: the efficiency of a perfectly reversible heat engine depends upon nothing other than the temperature of the boiler and the temperature of the condenser.
[John H. Lienhard, How Invention Begins (Oxford : Oxford University Press, 2006) p. 90]
Amazing conclusion #1: virtually everything about heat flowing from a warm place to a cool place depends only on the difference in temperature between the warm place and the cool place.
Amazing conclusion #2: there is an idea, call it entropy, that encapsulates #1. If we let 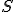 stand for entropy, and
stand for entropy, and 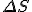 stand for the change in entropy, then we can write
stand for the change in entropy, then we can write
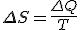
This is Clausius’ definition in symbols.
Now, there’s a lot of philosophical, interpretative baggage that travels with the idea of entropy, but if you can keep this simple approach in mind you can save a lot of heartburn pondering the deeper meaning of entropy and time’s arrow and the heat-death of the universe.
Entropy is an accounting tool. When heat flows between the hot place and the cold place, at best, if you allow it very carefully, you may be able to reverse the process but you will never do better, which means that you will never find the cold place getting colder than originally, nor the hot place getting hotter than originally, no matter what you do, unless you put still more heat into the system.
That’s one version of the notorious “Second Law of Thermodynamics”. There are a number of other forms.
For instance, another way of saying was I just said: entropy never decreases. There, thermodynamic accounting made easy.
Another one that’s useful: if you construct a device that uses heat flowing from a hot place to a cold place to do mechanical work — say, in a steam engine — some of the heat is always wasted, i.e., it goes into increasing entropy. Put another way: heat engines are never 100% efficient, not because we can’t build them but because it is physically impossible.
Think for a moment and you’ll see that the implication of this latter form of the Second Law of Thermodynamics is a statement that perpetual motion machines are impossible. They just are, not because a bunch of physicists though it might be a good idea to say it’s impossible, but because they are. That’s the way the universe is made.
Entropy needn’t be scary.
———-
* 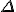 is a general purpose symbol often used to indicate a change in the quantity represented by the letter following it.
is a general purpose symbol often used to indicate a change in the quantity represented by the letter following it.
Feb
03
Posted by jns on
February 3, 2008
Recently I finished reading Jared Diamond’s The Third Chimpanzee : The Evolution and Future of the Human Animal (New York : HarperCollins Publishers, 1992, 407 pages). I quite enjoyed it. It’s the third of his books I’ve read. I previously enjoyed Collapse and Guns, Germs, and Steel, but I didn’t mind that this was a significantly shorter book. Here’s my book note.
In some ways this book rehearses arguments that will appear in the later, larger tomes in much more fleshed-out form, but it’s still its own book. This one’s theme is, more or less, an evolutionary look at what makes humans human. As usual, I found a few excerpts I wanted to share that didn’t quite fit into the note.
In a discussion of sexual selection, the subject of the human penis arises (if you’ll pardon the expression), and the glib answer would say something about the size of the penis’ being selected as a display, implying that the display is directed towards females. But, perhaps not….
While we can agree that the human penis is an organ of display, the display is intended not for women but for fellow men.
Other facts confirm the role of a large penis as a threat or status display toward other men. Recall all the phallic art created by men for men, and the widespread obsession of men with their penis size. Evolution of the human penis was effectively limited by the length of the female vagina: a man’s penis would damage a woman if it were significantly larger. Howerver, I can guess what the penis would look like if this practical constraint were removed and if men could design themselves. It would resemble the penis sheaths (phallocarps) used as male attire in some areas of New Guinea where I do fieldwork. Phallocarps vary in length (up to two feet), diameter (up to 4 inches), shape (curved or straight), angle made with the wearer’s body, color (yellow or red), and decoration (e.g., a tuft of fur at the end). Each man has a wardrobe of several sizes and shapes from which to choose each day, depending on his mood that morning. Embarrassed male anthropologists interpret the phallocarp as something used for modesty or concealment, to which my wife had a succinct answer on seeing a phallocarp: “The most immodest display of modesty I’ve ever seen!” [p. 76]
The discussion moves on to the curious case of concealed ovulation in humans, at least compared to our animal relatives.
So well concealed is human ovulation that we did not have accurate scientific information on its timing until around 1930. Before that, many physicians thought that women could conceive at any point in their cycle, or even that conception was most likely at the time of menstruation. In contrast to the male monkey, who has only to scan his surroundings for brightly swollen lady monkeys, the unfortunate human male has not the faintest idea which ladies around him are ovulating and capable of being fertilized. A woman herself may learn to recognize sensations associated with ovulation, but it is often tricky, even with the help of thermometers and ratings of vaginal mucus quality. Furthermore, today’s would-be mother, who tries in such ways to sense ovulation in order to achieve (or avoid) fertilization, is responding by cold-blooded calculation to hard-won, modern book knowledge. She has no other choice; she lacks the innate, hot-blooded sense of sexual receptivity that drives other female mammals.
Our concealed ovulation, constant receptivity, and brief fertile period in each menstrual cycle ensure that most copulations by humans are at the wrong time for conception. To make things worse, menstrual-cycle length varies more between women, or from cycle to cycle in a given woman, than for other female mammals. As a result, even young newlyweds who omit contraception and make love at maximum frequency have only a 28 percent probability of conception per menstrual cycle. Animal breeders would be in despair if a prize cow had such low fertility, but in fact they can schedule a single artificial insemination so that the cow has a 75 percent chance of being fertilized! [pp. 77--78]
Diamond has spent much of his research career among the people of New Guinea. He talks at length of “first contact”, the strange moment when two tribes of people discover each other, previously knowing nothing of their existence. Remarkable, before 1938, it was thought that the interior of New Guinea was unpopulated. The Archbold Expedition of 1938 unexpectedly found that the Grand Valley was populated by some 50,000 people. (There’s another excerpt about the Archbold Expedition in the book note.) What a shocker! But contact has its price. I found this story particularly poignant.
Take artistic diversity as one obvious example. Styles of sculpture, music, and dance used to vary greatly from village to village within New Guinea. Some villagers along the Sepik River and in the Asmat swamps produced carvings that are now world-famous because of their quality. But New Guinea villagers have been increasingly coerced or reduced into abandoning their artistic traditions. When I visited an isolated tribelet of 578 people at Bomai in 1965, the missionary controlling the only store had just manipulated the people into burning all their art. Centuries of unique cultural development (“heathen artifacts,” as the missionary put it) had thus been destroyed in one morning. [p. 231]
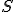 stand for entropy, and
stand for entropy, and 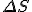 stand for the change in entropy, then we can write
stand for the change in entropy, then we can write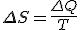
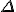 is a general purpose symbol often used to indicate a change in the quantity represented by the letter following it.
is a general purpose symbol often used to indicate a change in the quantity represented by the letter following it.